AL JAZEERA BOLTS INDUSTRIES LLC - | E-Showroom

AL JAZEERA BOLTS INDUSTRIES LLC - | E-Showroom
AL JAZEERA BOLTS INDUSTRIES LLC
SAJAA INDUSTRIAL AREA NEAR SAJ GAS PLANT SHARJAH UAE Sajaa Industrial Area Sharjah UAE Sajaa Industrial Area 3.2 Km off Gas Plant Road Sharjah
United Arab Emirates

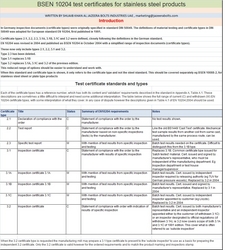
Applications Of Stainless Steel
Algeria Yellow Pages Online is a Local Business to Business Directory in Algeria offering business list of more than 250,000 companies. You can find Hotels in Algeria , Companies in Algeria , Properties in Algeria , Travel info in Algeria through this Site. Yellow Pages Algeria Updated in 2026 Get Maximum Benefit for your Business Visit YP MarketPlaces
| About Us Careers Company Information User Guide About Us |
Buying Options Post Buying Leads Browse Categories Companies in Algeria How to Buy |
Selling Options Post Selling Leads Browse Categories How to Sell |
Safety & Support Help Safety & Security Copyright Infringment |
Advertising How to Advertise? Host Website with us Elite Membership |
Method Of Payment Privacy Policy Refund Policy Dispute & Resolution Policy Terms |
| Thanks for Posting your Requirement
with
Algeria Yellow Pages Online
If you are not Verified Buyer then Please Verify Your Email to get Quotes from Verified Suppliers. |








|
Thanks for Reporting Error in Listing of on Algeria Yellow Pages Online
Our Technical Team will review the Information and will Rectify the Error as Soon as Possible. |
| Thanks for Reply.
Algeria Yellow Pages Online
Your Reply is Sent to the Buyer. |
| Thanks for Reply.
Your Reply is Sent to the Seller. |
| Ok Close |